Marketing Chen
Marketing Cai
Recently, Professor Wen Zhenhai from the Fujian Institute of Research on the Structure of Matter (FJIRSM), Chinese Academy of Sciences, along with Professors Fan Zhuangjun and Huang Yichao from China University of Petroleum (East China), and Associate Professor Gao Wenpei from Shanghai Jiao Tong University, jointly developed a Pt/Mo₂N-NrGO electrocatalyst that exhibits excellent performance in the hydrogen evolution reaction (HER) during alkaline water electrolysis.
The Pt nanoclusters and Mo₂N support are interconnected through Pt–N–Mo bonds at the interface. This interfacial electronic coupling facilitates the co-catalysis of H₂O decomposition into Pt–H* and Mo–OH* by Pt and Mo, thereby enhancing the reaction kinetics of the rate-determining step in alkaline water electrolysis. Techno-economic analysis indicates that the hydrogen production cost of this catalyst is only $2.02 per kilogram, meeting DOE standards, and it holds broad application prospects in industrial anion-exchange membrane water electrolyzers (AEMWE). This study, titled "A Strongly Coupled Cluster Heterostructure with Pt–N–Mo Bonding for Durable and Efficient H₂ Evolution in Anion-Exchange Membrane Water Electrolyzers," was published in Nano-Micro Letters.

In this study, silicon nitride thin films (developed by YW MEMS (Suzhou) Co., Ltd.) were employed as TEM supports for characterization. The catalyst powder was dispersed in anhydrous ethanol to form an ink, which was then drop-cast onto the silicon nitride film for TEM, STEM, and EDS analysis.
Construction of a Strongly Coupled Heterostructure for Enhanced Catalysis
Constructing a strongly coupled heterostructure with high catalytic activity is crucial for improving catalytic reactions, especially those involving multiple intermediates. The authors used platinum-containing Anderson-type polyoxometalates (POMs) loaded onto polyaniline-modified graphene as a precursor. The protonated polyaniline, carrying a positive charge, electrostatically interacts with the negatively charged POMs, ensuring robust loading onto the graphene support and preventing particle sintering and agglomeration during high-temperature processes. Through high-temperature synthesis, a Pt/Mo₂N-NrGO heterostructure was obtained, where strongly coupled Pt/Mo₂N nanoclusters are supported on nitrogen-doped reduced graphene oxide (NrGO).
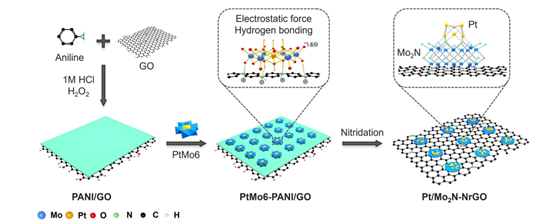
Figure 1: Schematic illustration of the synthesis route for Pt/Mo₂N-NrGO.
Morphological and Structural Characterization
Using silicon nitride thin films as TEM supports, the authors characterized the catalyst's morphology. HAADF-STEM images revealed that Pt/Mo₂N nanoclusters (~2.0 nm in diameter) are uniformly embedded in NrGO nanosheets. Most Pt species exist as intermetallic nanoclusters bonded with Mo₂N. EDS mapping further confirmed the strong interfacial coupling between Pt and Mo₂N, while the Pt content (only 3.39%) is significantly lower than that of commercial Pt/C catalysts.
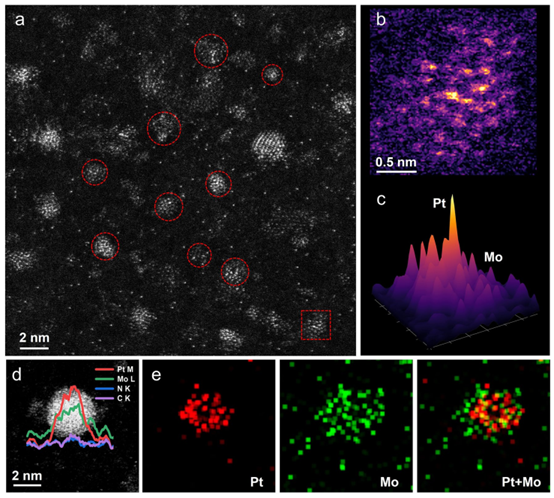
Figure 2: Morphological characterization of Pt/Mo₂N-NrGO.
(a) Aberration-corrected HAADF-STEM image (red circles indicate Pt/Mo₂N clusters).
(b) 2D intensity distribution of the red dashed box in (a).
(c) 3D surface intensity map of (b).
(d) Magnified aberration-corrected HAADF-STEM image of a single cluster with linear intensity profiles.
(e) Corresponding STEM-EDS elemental mapping of the Pt/Mo₂N-NrGO cluster in (d).
XRD and XPS Analysis
The XRD pattern of Pt/Mo₂N-NrGO shows characteristic peaks at 37.30°, 42.76°, 62.55°, and 75.73°, corresponding to the (111), (200), (220), and (311) planes of cubic Mo₂N (PDF#73-1768). Weak peaks at 39.75°, 46.16°, 67.31°, and 79.23° are attributed to the (111), (200), (220), and (311) planes of Pt (#04-0802). The main peak at 37.30° (vs. 35.57° for Mo₂N-NrGO) indicates lattice expansion due to Pt incorporation (tensile strain). Compared to Pt-NrGO, the weaker Pt signals in Pt/Mo₂N-NrGO suggest that Mo₂N effectively suppresses Pt aggregation. Additionally, the broader and lower Mo₂N peaks in Pt/Mo₂N-NrGO confirm that PtMo₆ units prevent Mo₂N agglomeration.
XPS reveals two oxidation states for Pt: Pt⁰ (72.05 eV, 75.35 eV) and Pt⁴⁺ (72.52 eV, 75.90 eV). The Pt⁴⁺/Pt⁰ ratio increases from 0.50 (Pt-NrGO) to 1.16 (Pt/Mo₂N-NrGO), indicating easier oxidation of Pt on Mo₂N. High-resolution Mo 3d spectra show peaks at 228.48 eV and 231.48 eV (Mo–N), 229.48 eV and 233.78 eV (MoO₂), and 232.58 eV and 235.78 eV (MoO₃). The MoOₓ/Mo₂N ratio decreases from 0.65 (Mo₂N-NrGO) to 0.59 (Pt/Mo₂N-NrGO), confirming electron transfer from Pt to Mo₂N, which enhances Pt–H* adsorption and lowers the Tafel barrier.
Pt L₃-edge XANES shows an average Pt oxidation state of +1.45 (higher than Pt⁰), consistent with XPS. FT-EXAFS confirms Pt–N–Mo bonding at the interface, with a Pt–N peak at 1.56 Å (shorter than PtO₂’s Pt–O bond, 1.66 Å), indicating direct Pt–Mo linkage via N.
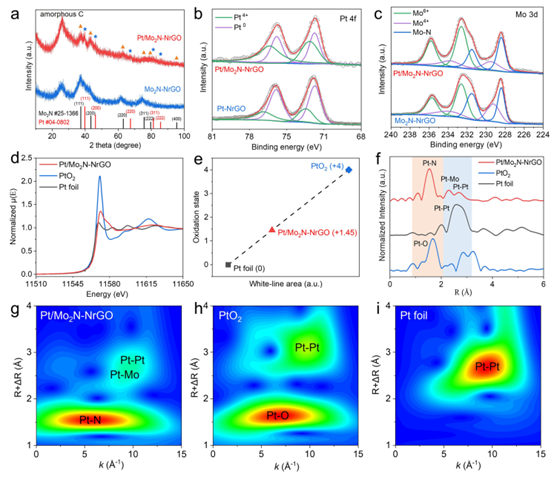
Figure 3: Structural characterization of Pt/Mo₂N-NrGO.
(a) XRD patterns of Pt/Mo₂N-NrGO and Mo₂N-NrGO.
(b) High-resolution Pt 4f and (c) Mo 3d XPS spectra.
(d) Normalized Pt L₃-edge XANES spectra.
(e) Fitted average Pt oxidation state.
(f) EXAFS spectra.
(g–i) Wavelet transforms of Pt/Mo₂N-NrGO, PtO₂, and Pt foil.
Electrocatalytic Performance in Alkaline HER
Evaluated via a standard three-electrode system, Pt/Mo₂N-NrGO exhibits an ultralow overpotential of 11 mV at 10 mA cm⁻², surpassing Mo₂N-NrGO, Pt-NrGO, NrGO, and commercial Pt/C. Its Tafel slope (31 mV dec⁻¹) is much lower than that of Mo₂N-NrGO, Pt-NrGO, and Pt/C, indicating a Volmer-Tafel mechanism. The catalyst achieves a mass activity of 17.5 A mg⁻¹ and TOF of 17.82 H₂ s⁻¹, 10× higher than Pt/C.
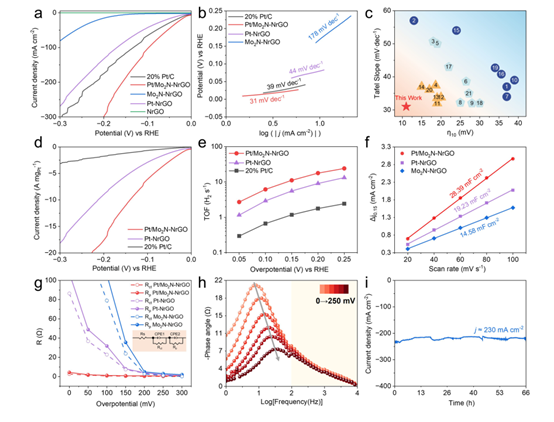
Figure 4:
(a) LSV curves of 20% Pt/C, Pt/Mo₂N-NrGO, Pt-NrGO, Mo₂N-NrGO, and NrGO.
(b) Tafel plots.
(c) Comparison of Tafel slopes and overpotentials at η₁₀.
(d) Mass activity.
(e) TOF.
(f) Double-layer capacitance.
(g) EIS analysis (inset: equivalent circuit).
(h) Bode phase plots.
(i) Chronoamperometry at 180 mV vs. RHE.
Practical Application in AEMWE
Optimized Pt/Mo₂N-NrGO was tested as a cathode catalyst in AEMWE (1.0 M KOH, 25–80°C) with NiFe LDH as the cathode and Piperion AEM-a40 as the membrane. At 80°C, Pt/Mo₂N-NrGO||NiFe LDH achieves 1.0 A cm⁻² at 1.66 V and 2.0 A cm⁻² at 1.84 V, outperforming most reported AEMWE catalysts. After 500 hours at 1.5 A cm⁻², only a slight potential increase is observed, demonstrating exceptional durability. Techno-economic analysis confirms a hydrogen production cost of $2.02/kg, meeting DOE standards.
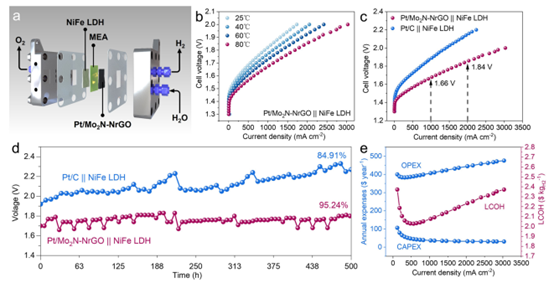
Figure 5: AEMWE performance and techno-economic analysis (TEA).
(a) AEMWE schematic.
(b) Polarization curves at different temperatures.
(c) Comparison with commercial Pt/C.
(d) Stability test at 1.5 A cm⁻².
(e) TEA at varying current densities.
Synergistic Catalytic Mechanism
In situ Raman spectroscopy reveals dynamic evolution during HER: peaks at 795.5 cm⁻¹ (Mo–OH stretch) and 2328.5 cm⁻¹ (Pt–H stretch) intensify, confirming H adsorption on Pt and OH binding on Mo. DFT calculations (Pt/Mo₂N, Pt(111), Mo₂N(111)) show that the Pt–N–Mo interface facilitates water dissociation via a "scissor-like" mechanism, while Mo₂N provides OH* adsorption sites to prevent Pt–OH formation and maintain hydrophilic pathways.
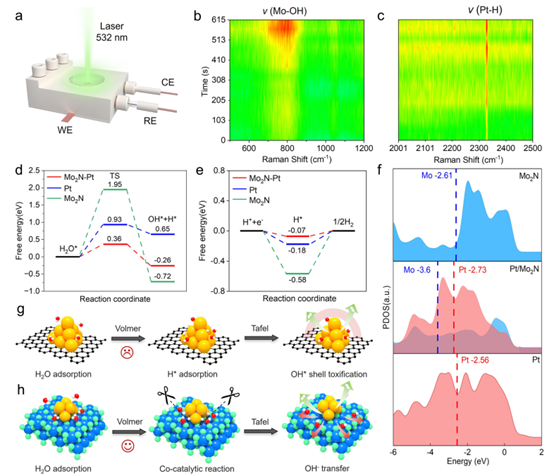
Figure 6: Synergistic catalysis mechanism.
(a) In situ Raman setup.
(b) Raman spectra during HER.
(d–e) Gibbs free energy diagrams for Volmer and Tafel steps.
(f) DOS analysis of Mo₂N(111), Pt/Mo₂N-NrGO, and Pt(111).
(g–h) Proposed mechanisms for Pt/C and Pt/Mo₂N-NrGO.
Acknowledgments
This work was supported by the National Natural Science Foundation of China, Shandong Taishan Scholars Program, Shandong Natural Science Foundation, and Fundamental Research Funds for the Central Universities.