Marketing Chen
Marketing Cai

Nowdays, Professor Mai Liqiang research group in International School of Materials Science and Engineering and State Key Laboratory of Advanced Technology for Materials Synthesis and Processing from Wuhan University of Technology. They use micro-device to explore the continuous electric conductance evolution of lattice P-doped oxides during the electrochemical activation process. To study and demonstrate the induced electronic coupling between new-formed oxides and P-O groups. The coupled P–O groups effectively promote the metal–oxygen covalency of new-formed oxides, which accelerates electron transfer between active metallic center and oxygen adsorbates, thus leading to the enhanced electrocatalytic activity. The team believes such unique on-chip electrochemical microdevice platform can also be applied in other related fields to understand the dynamic behavior of energy materials at nanoscale. Related achievements were published in Nano Energy with the title of "Unveiling the role of surface P–O group in P-doped Co3O4 for electrocatalytic oxygen evolution by On-chip micro-device".
Transition metal phosphides or partially phosphatized oxides usually suffer from surface reconstruction during oxygen evolution reaction (OER), but still possess enhanced catalytic activity than directly synthesized oxides, which has aroused great interest in exploring the causes of such high catalytic activity. To monitor electronic property of catalyst during the OER can provide crucial insights into catalytic ability.
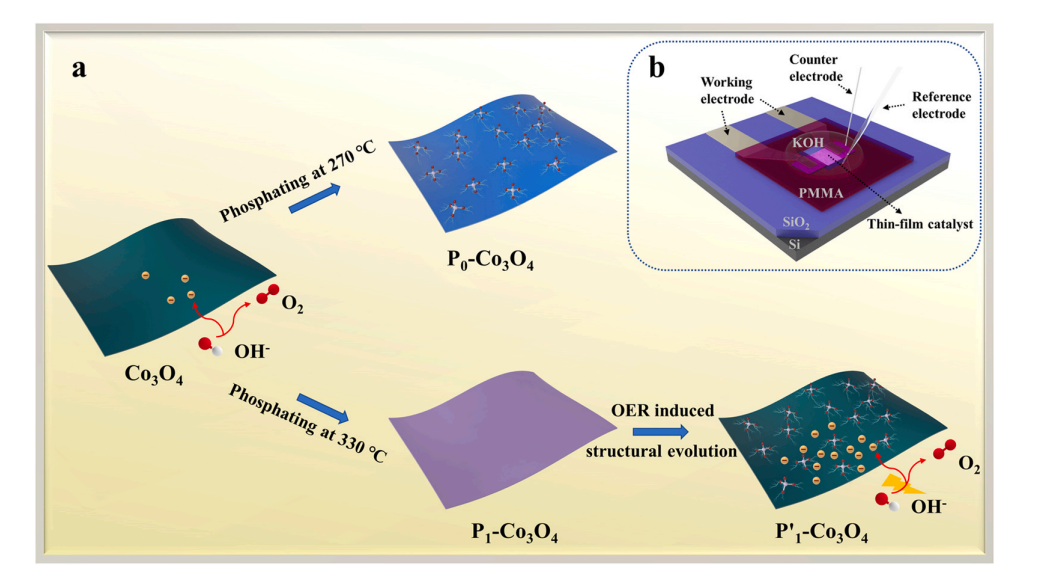
(a)Schematic diagram of P–O groups induced OER activity enhancement. (b) Schematic of the individual thin-film-based planar electrochemical micro device.
By designing a planar electrochemical microdevice based on individual P1-Co3O4 thin-films, we for the first time measure the electric conductance of reconstructed lattice phosphorus doped Co3O4, and establish the correlation between intrinsic conductance and electrochemical activity of lattice phosphorus doped Co3O4 during the reconstruction, clearly recognizing the influence of intrinsic conductance on catalytic activity of P-doped Co3O4.Moreover, based on such unique microdevice platform, the real-time resistance of reconstructed lattice phosphorus doped Co3O4 during the OER is monitored by in situ I-V measurement.Combining with on-chip EIS measurement at 1.6 V vs RHE, it reveals that the reconstructed lattice phosphorus doped Co3O4 has a faster charge transfer kinetics than the pristine synthesized Co3O4 at high electrochemical potential.
In addition, this study suggest that unique on-chip electrochemical microdevice platform can also be applied in other related fields to understand the dynamic behavior of energy materials at nanoscale.
To elucidate the crystal structure of the P′1-Co3O4, the HRTEM analysis was carried out. The silicon nitride support film used for the characterization of Co3O4 and P′1-Co3O4 crystal structure was from YW MEMS CleanSiN. CleanSiN is an independently developed and produced silicon nitride membrane brand by YW MEMS. The thinnest silicon nitride membrane produced by YW MEMS can be up to 10 nm, with good mechanical strength, high temperature resistance and surface thermal conductivity under the premise of ensuring super smooth and super clean quality requirements. This study makes full use of these excellent features of silicon nitride membrane to do characterization of Co3O4 and P′1-Co3O4 crystal structure in TEM.
Original link:https://doi.org/10.1016/j.nanoen.2021.105748